Important note: Information on this page was accurate at the time of publication. This page is no longer being updated.
Understanding the Results of VOICE
About VOICE
-
VOICE – Vaginal and Oral Interventions to Control the Epidemic – was a major HIV prevention trial that tested whether antiretroviral (ARV) medicines commonly used to treat people with HIV are safe and effective in preventing sexual transmission of HIV in women. The study focused on two different ARV-based approaches: daily use of an ARV tablet – an approach called oral pre-exposure prophylaxis, or PrEP – and daily use of a vaginal microbicide containing an ARV in gel form. Specifically, VOICE tested the safety and effectiveness of three different products: an oral tablet containing tenofovir (known by the brand name Viread®); an oral tablet that contains both tenofovir and emtricitabine (known as Truvada®); and tenofovir gel, a vaginal microbicide formulation of tenofovir.
-
VOICE began in September 2009 and enrolled 5,029 women in Uganda, South Africa and Zimbabwe, with follow-up of all participants completed in August 2012. The study’s primary results were reported at the Conference on Retroviruses and Opportunistic Infections (CROI) in Atlanta, March 4, 2013.
Key Points About The Results of VOICE
-
Of the three products tested in VOICE – tenofovir gel, oral tenofovir and oral Truvada – none proved to be effective among the women enrolled in the trial; most participants did not use them daily as recommended.
-
Although the results of VOICE are disappointing, VOICE has in fact provided a clear answer: daily use of a product – whether a vaginal gel or an oral tablet – is not the right HIV prevention approach for African women like those in VOICE, who were predominately young and unmarried.
-
Compared to older, married participants, single women under the age of 25 were least likely to use their assigned products and most likely to acquire HIV. Incidence of HIV in these young women exceeded 10 percent at some trial sites in South Africa, a rate considerably higher than expected and underscoring the gravity of the epidemic in a population that continues to be among the most vulnerable for acquiring HIV.
-
Although Truvada was found to be effective in other trials in other populations, and was subsequently approved by the U.S. Food and Drug Administration (FDA) for HIV prevention, the results of VOICE are consistent with the FEM-PrEP study, which involved a very similar population of women and did not find Truvada effective. As in VOICE, most of the participants in FEM-PrEP did not follow the daily regimen.
-
Data from VOICE will be important as the World Health Organization (WHO) considers the evidence from different clinical trials and demonstration projects in its development of formal implementation guidelines on the use of oral PrEP, which it expects to release in 2015.
-
VOICE highlights the urgent need for safe, effective and practical HIV prevention methods that young, unmarried women will actually use. Approaches that offer long-acting protection may be more suitable for the population of women in VOICE. Two ongoing trials – ASPIRE and The Ring Study – are evaluating a vaginal ring containing the ARV drug dapivirine that women use for a month at a time.
Background
-
As a flagship trial of the Microbicide Trials Network (MTN), VOICE was funded by the National Institute of Allergy and Infectious Diseases (NIAID) with co-funding from the National Institute of Mental Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, all part of the U.S. National Institutes of Health (NIH). The study was led by Zvavahera Mike Chirenje, M.D., from the University of Zimbabwe in Harare; and Jeanne Marrazzo, M.D., M.P.H., from the University of Washington in Seattle. As co-sponsors of the trial, CONRAD and Gilead Sciences, Inc. provided the study products.
-
VOICE was conducted at 15 NIAID-funded clinical research sites in Uganda, South Africa and Zimbabwe and enrolled 5,029 sexually active HIV-negative women. Of these women, 4,077 were from South Africa, 322 from Uganda and 630 were from Zimbabwe. Nearly half of the participants were younger than age 25 (mean age was 25.3) and most (79 percent) were unmarried.
-
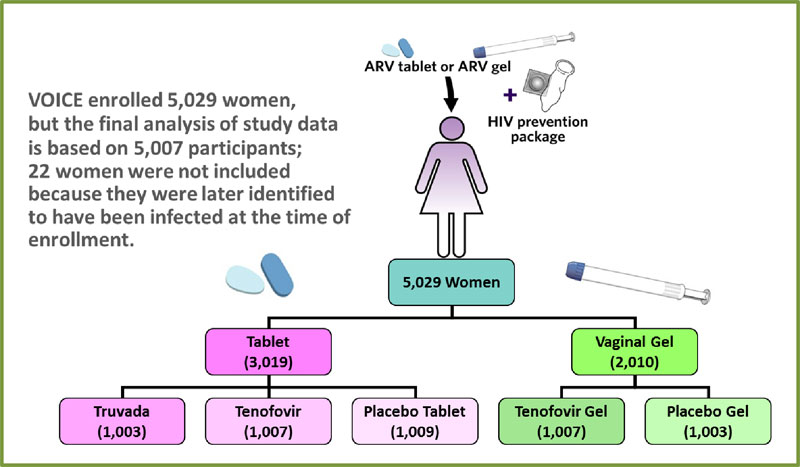 VOICE was designed as a large Phase IIb (proof of concept) trial to evaluate the safety and effectiveness of a daily tablet (tenofovir or Truvada) or of a vaginal gel (tenofovir gel) for preventing the sexual transmission of HIV in women. Learning about the safety and effectiveness of each approach required the kind of trial in which participants were randomly assigned to different study groups, including groups that used a placebo, which has no active drug. Because the study was “blinded,” neither participants nor researchers knew who was in which group during the trial. Like all HIV prevention trials, VOICE participants received ongoing HIV risk reduction counseling, condoms and diagnosis and treatment of sexually transmitted infections – standard methods for reducing HIV risk – throughout their participation.
VOICE was designed as a large Phase IIb (proof of concept) trial to evaluate the safety and effectiveness of a daily tablet (tenofovir or Truvada) or of a vaginal gel (tenofovir gel) for preventing the sexual transmission of HIV in women. Learning about the safety and effectiveness of each approach required the kind of trial in which participants were randomly assigned to different study groups, including groups that used a placebo, which has no active drug. Because the study was “blinded,” neither participants nor researchers knew who was in which group during the trial. Like all HIV prevention trials, VOICE participants received ongoing HIV risk reduction counseling, condoms and diagnosis and treatment of sexually transmitted infections – standard methods for reducing HIV risk – throughout their participation. -
VOICE originally had five study groups – two gel groups (tenofovir gel and a placebo gel) and three tablet groups (tenofovir, Truvada and a placebo tablet) – with about 1,000 women in each group who were asked to use their assigned study product every day. In late 2011, VOICE stopped testing tenofovir tablets and tenofovir gel after separate routine reviews of study data by an independent Data Safety and Monitoring Board determined that while each was safe, neither was effective in preventing HIV compared to the matched placebos among the women in those groups. VOICE continued to evaluate Truvada until the scheduled end of the study in August 2012.
Results of VOICE
Results from the study’s primary data analysis – the answers to the study’s main questions about safety and effectiveness – include the following:
-
Of the 5,029 women enrolled in VOICE, 312 acquired HIV during the study (another 22 women who were later identified as being infected at enrollment were excluded from the analysis), for an overall HIV incidence of 5.7 percent, nearly twice what investigators had expected when they designed the trial. HIV incidence, which represents the number of women who become newly infected for every 100 participants in a given year, ranged from 0.8 percent in Zimbabwe, to 2.1percent in Uganda, to 7 percent in South Africa. The incidence was nearly 10 percent at some South African trial sites.
-
Adherence to product use was low across all groups, according to an analysis of blood samples from a subset of 773 participants (including 185 women who acquired HIV): drug was detected in 29 percent of the blood samples from women in the Truvada group, 28 percent of the blood samples of those in the oral tenofovir group and 23 percent among those in the tenofovir gel group. In sharp contrast, adherence to product use was calculated to be about 90 percent based on what the participants themselves had reported to trial staff and on monthly counts of unused gel applicators and leftover pills.
-
Compared to both older and married participants, young, unmarried women were much less likely to use their study products and much more likely to acquire HIV. In the Truvada group, for example, drug was detected in just 21 percent of blood samples of younger, single women compared to 54 percent of those married and over age 25.The overall HIV incidence was 8.8 percent for unmarried women under age 25 compared to 0.8 percent for older women who were married, differences that were statistically significant.
-
No safety concerns were identified for any of the products. However, compared to their matched placebos, no product was effective in reducing the risk of HIV. Effectiveness was calculated based on data from 5,007 participants.
-
-
61 of 994 women in the Truvada group acquired HIV (4.7 percent HIV incidence)
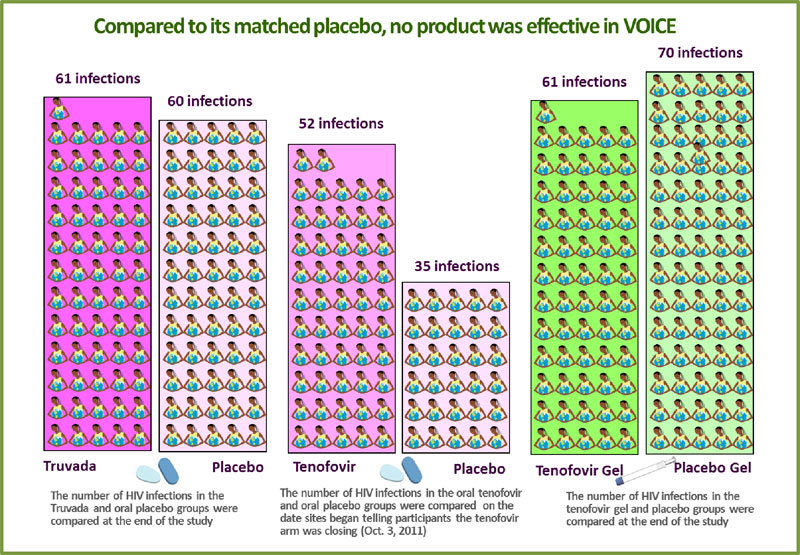 compared to 60 of 1,008 in the oral placebo group (4.6 percent incidence).
compared to 60 of 1,008 in the oral placebo group (4.6 percent incidence). -
Of the 1,002 women in the oral tenofovir group, 60 acquired HIV. Incidence, however, was calculated to reflect what had occurred up until Oct. 3, 2011, when sites began informing participants that testing of oral tenofovir was to stop. At this time, there were 52 infections in the tenofovir tablet group and 35 in the oral placebo group, for HIV incidence rates of 6.3 and 4.2 percent, respectively.
-
Of the 1,003 women assigned to use tenofovir gel, 61 women acquired HIV (5.9 percent HIV incidence), and 70 of the 1,000 women in the placebo gel group became infected (6.8 percent HIV incidence). Though the estimates of effectiveness for both oral tenofovir and Truvada were less than zero, tenofovir gel was estimated to reduce the risk of HIV by 14.7 percent compared to the placebo gel (there were 14.7 percent fewer infections in that group), but with a confidence interval indicating the level of effectiveness could be between -21 percent and 40 percent, this was not a statistically significant finding.
-
Because massive amounts of data were collected in VOICE as well as in VOICE sub-studies, results of these and other analyses are not expected to be available until the coming months or later in the year.
- Results from two qualitative behavioral studies, VOICE C and VOICE D, will help to clarify why women did or did not use the products. VOICE-C, the Community and Adherence Sub-study, aims to identify community-based factors and beliefs that may have influenced women’s ability and willingness to follow the daily regimens tested in VOICE, while VOICE-D hopes to understand individual behaviors, beliefs and attitudes about HIV risk that may have had an impact on women’s adherence to product use during VOICE, and, in turn, the study’s ability to demonstrate product efficacy. VOICE-D is enrolling 80 former VOICE participants and involves a single in-depth interview with a researcher not otherwise connected to VOICE or any trial site.
- Examination of drug levels in vaginal fluid should provide greater insight into the relationship between product use and product efficacy, particularly for tenofovir gel. In addition, a secondary analysis of blood samples hopes to provide information about the potential of the study products—especially tenofovir gel—to reduce the risk of acquiring herpes simplex virus (HSV).
- Results of the Bone Density Sub-study (VOICE-B) may be important for understanding the effects, if any, that oral tenofovir and Truvada have on bone health in HIV-negative women, and in particular, pre-menopausal women in Africa. Such data will help inform potential rollout of ARV-based approaches for prevention of HIV. VOICE-B involves 518 women from Uganda and Zimbabwe. Participants are being followed until August 2013, and results are expected before the end of the year.
- VOICE will also provide information on incidence of HIV drug resistance in women who acquired HIV during VOICE and on the potential relationship between the use of hormonal contraceptives and HIV risk.
Context and Implications
Tenofovir Gel
In July 2010, the CAPRISA 004 study found tenofovir gel was safe and reduced the risk of HIV by 39 percent among women who used it before and after sex compared to women who used a placebo gel, a finding that was considered a major milestone for the field. (CAPRISA 004 also unexpectedly found that tenofovir gel also reduced the risk of HSV-2 by 51 percent, the first time that any kind of biomedical prevention method was shown to be effective against HSV-2.) But the study was relatively small (889 women from one region in South Africa) and its results included a wide confidence interval that in statistical terms meant the true level of effectiveness of tenofovir gel – when used before and after sex – could be anywhere between 6 and 60 percent. More data from a larger trial would be needed, and the U.S. FDA indicated that along with the results of CAPRISA 004, it would review data from VOICE, testing the gel’s daily use, as the second pivotal trial to support possible licensure of tenofovir gel. Subsequently, when a routine interim review of study data during VOICE determined that the gel wasn’t effective in preventing HIV, hope shifted to FACTS 001. FACTS 001 is an ongoing Phase III trial of the same regimen tested in CAPRISA 004 (before and after sex) that plans to enroll 2,900 women at nine South African sites, with results expected in 2015. Also ongoing is CAPRISA 008, an open-label follow-on study for former CAPRISA 004 participants that aims to determine the feasibility of gel delivery through family planning services.
As a co-licensee for tenofovir gel, CONRAD has been leading all discussions with drug regulatory authorities, including the FDA and the South African Medicines Control Council in support of the gel’s possible approval. In addition to data from CAPRISA 004 and FACTS 001, the FDA has indicated it will review the VOICE results and data from a large portfolio of studies conducted by both the MTN and CONRAD, including two studies that the MTN is currently conducting. One is a Phase II trial looking at safety and drug absorption in pregnant and breastfeeding women (MTN-008), while the other is a Phase I study examining how vaginal intercourse may affect drug absorption and drug activity (MTN-011).
In other MTN studies, researchers are evaluating a reduced glycerin formulation of tenofovir gel. In one trial to be conducted in South Africa and the United States, researchers will examine drug absorption patterns in both rectal and vaginal tissue when the gel is applied either vaginally or rectally (MTN-014), while a Phase II trial, MTN-017, hopes to determine whether the reformulated gel is safe and acceptable as a rectal microbicide among men who have sex with men (MSM) and transgender women in Peru, South Africa, Thailand and the United States. MTN-017, which is the first Phase II trial of a rectal microbicide, is expected to begin mid-2013.
Truvada
The U.S. FDA’s approval of Truvada for HIV prevention, granted in July 2012, was based primarily on the results of two pivotal studies involving two different populations. The Partners PrEP Study involved 4,758 heterosexual couples in which one partner has HIV, while the iPrEx study enrolled 2,500 MSM. In Partners PrEP, which tested both tenofovir and Truvada, there were 75 percent fewer infections among those who took Truvada compared to placebo, while in iPrEx, Truvada was associated with a 42 percent reduction in HIV risk. A smaller trial of 1,200 heterosexual men and women in Botswana found a 62.6 percent reduction in HIV among those assigned to take Truvada.
These same studies also demonstrated that Truvada was more effective in protecting against HIV when the daily regimen was followed consistently. Indeed, Truvada was not effective in the FEM-PrEP Study, and many of its participants, 2,119 women from Kenya, South Africa and Tanzania, did not follow the daily pill-taking regimen as instructed.
In July 2012, the World Health Organization (WHO) issued guidance on PrEP for serodiscordant couples (in whom one partner is HIV-infected) and MSM, recommending its use only in the context of demonstration projects. WHO expects to issue formal PrEP implementation guidelines in 2015 that will consider emerging evidence from trials such as VOICE, as well as outcomes of in-country demonstration projects. Information about Truvada and its “real world” use is being collected in open-label trials, such as iPrEx OLE and the Partners Demonstration Project, and in several other demonstration projects and implementation studies taking place in the United States.
Oral Tenofovir
The Partners PrEP Study of serodiscordant couples found tenofovir reduced the risk of HIV by 67 percent compared to a placebo. Other than VOICE, only one other trial has evaluated daily use of oral tenofovir for preventing HIV. The Bangkok Tenofovir Study involved 2,400 injection drug users in Thailand, with results expected later in 2013.
Current HIV Prevention Trials in Women
There are three ongoing Phase III HIV prevention trials specifically focused on women. In addition to FACTS testing tenofovir gel used before and after sex, ASPIRE and The Ring Study are assessing whether a vaginal ring containing the ARV drug dapivirine is safe and effective for protecting against HIV when used by women for a month at a time. The dapivirine ring is the first ARV-based product to enter efficacy testing that is intended for monthly use and the first involving an ARV other than tenofovir or a tenofovir combination. ASPIRE – A Study to Prevent Infection with a Ring for Extended Use – is being conducted by the MTN. The Ring Study is being conducted by the International Partnership for Microbicides (IPM), which developed the dapivirine ring. More than 5,000 women from Africa will take part in these sister studies. Results of both are expected late 2014 or early 2015.
# # #
More information about VOICE can be found at http://www.mtnstopshiv.org/news/studies/mtn003. A summary of other oral PrEP and microbicide studies can be found at www.avac.org.
About the MTN
The Microbicide Trials Network (MTN) is an HIV/AIDS clinical trials network established in 2006 by the National Institute of Allergy and Infectious Diseases with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, all components of the U.S. National Institutes of Health. Based at Magee-Womens Research Institute and the University of Pittsburgh, the MTN brings together international investigators and community and industry partners who are devoted to preventing or reducing the sexual transmission of HIV through the development and evaluation of products applied topically to mucosal surfaces or administered orally.
Click here for PDF version of the document
4-March-2013
See Also
Daily HIV Prevention Approaches Didn’t Work for African Women in the VOICE Study
Mar 4, 2013
Nov 25, 2011
Questions and Answers on Decision to Modify VOICE, Outcome of November 17 DSMB Review
Nov 25, 2011
Sep 28, 2011
Questions and Answers on Decision to Modify VOICE
Sep 28, 2011
HIV Prevention Trial Milestone: VOICE Study Completes Enrollment of 5,000 Women
Jun 6, 2011
Apr 18, 2011
Nov 23, 2010
Results of CAPRISA 004 a turning point for HIV prevention, say MTN researchers conducting VOICE
Jul 19, 2010
VOICE Study, a major HIV prevention trial for women, is launched in Zimbabwe
Sep 16, 2009
HIV prevention trial of ARV-based strategies to begin next month
Jul 20, 2009
