Decision to Modify VOICE: Outcome of November 17 Data and Safety Monitoring Board (DSMB) Review of VOICE
About VOICE and the DSMB Review Outcome
1. What has been the aim of the VOICE Study?
VOICE – Vaginal and Oral Interventions to Control the Epidemic – is a major HIV prevention trial designed to test whether antiretroviral (ARV) medicines commonly used to treat people with HIV are safe and effective in preventing sexual transmission of HIV in women. The study has focused on two different ARV-based approaches: daily use of an ARV tablet – an approach called oral pre-exposure prophylaxis, or PrEP – and daily use of a vaginal microbicide containing an ARV in gel form. VOICE was the first effectiveness study of a vaginal microbicide that women use every day, and the only trial evaluating both an oral tablet and a vaginal gel in the same study. This design helps answer how each product works compared to its control (placebo gel or placebo tablet) and which approach women prefer.
Specifically, VOICE was designed to determine the safety and effectiveness of three different products: an oral tablet containing tenofovir; an oral tablet that contains both tenofovir and emitricitabine (known as Truvada®), and tenofovir gel, a vaginal microbicide with the same active ingredient as the oral tenofovir tablet. Oral tenofovir, known by the brand name Viread®, and Truvada, are both approved for use in the treatment of HIV. The trial is being conducted in Africa, where effective HIV prevention methods are critically needed, especially for women, who represent nearly 60 percent of adults living with HIV on that continent.
2. Who is conducting the study?
The VOICE Study is being conducted by a team of researchers working in the Microbicide Trials Network (MTN), an HIV/AIDS clinical trials network funded by the Division of AIDS (DAIDS) at the National Institute of Allergy and Infectious Diseases (NIAID) with co-funding from the National Institute of Mental Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, all components of the U.S. National Institutes of Health (NIH). Leading the study are Zvavahera Mike Chirenje, M.D., from the University of Zimbabwe in Harare; and Jeanne Marrazzo, M.D., M.P.H., from the University of Washington in Seattle. As co-sponsors of the trial, CONRAD of Arlington, Virginia, and Gilead Sciences, Inc., of Foster City, California, are providing the study products for free.
3. Who is participating in the VOICE Study, and where is it being conducted?
VOICE is being conducted at 15 NIAID-funded clinical research sites in South Africa, Uganda and Zimbabwe, where 5,029 sexually active HIV-negative women were enrolled: 4,077 in South Africa; 322 in Uganda; and 630 in Zimbabwe. The participants in VOICE are predominately women in their 20’s (and younger) who may or may not be married or in a committed relationship. Many are living in communities where HIV incidence is among the highest anywhere in the world.
4. When did the trial begin and how long is it planned to last?
The study began in September 2009 and completed participant enrollment in June 2011. As currently planned, the last participants in VOICE will have completed all study visits and follow-up by June or July 2012. The final results of the study are expected to be available late 2012 or early 2013.
5. How was VOICE originally designed and what has now changed?
VOICE was designed as a large Phase IIb (proof of concept) trial to evaluate two antiretroviral (ARV)-based approaches for preventing the sexual transmission of HIV in women – daily use of an ARV tablet (tenofovir or Truvada®) or a vaginal gel (tenofovir gel).
Learning about the safety and effectiveness of each approach required the kind of trial in which participants are randomly assigned by chance to different study groups, including groups that use a placebo, which has no active drug. Moreover, neither participants nor researchers know who is in which group while the study is ongoing because it is “blinded.” As with other HIV prevention trials, all participants in VOICE receive ongoing HIV risk reduction counseling, condoms and diagnosis and treatment of sexually transmitted infections (STIs) – standard measures for preventing HIV – throughout their participation.
VOICE originally had five study groups – two gel groups (tenofovir gel and an inactive placebo gel) 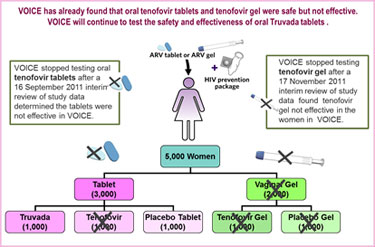 and three tablet groups (tenofovir, Truvada and an inactive placebo tablet) – with about 1,000 women in each group who were asked to use their assigned study product every day. In early October 2011, VOICE stopped testing the oral tenofovir tablet because a routine review of study data by the trial’s independent data safety and monitoring board (DSMB) determined that although the oral tenofovir tablets were safe, they were no better than a placebo in preventing HIV in the women assigned to that study group.
and three tablet groups (tenofovir, Truvada and an inactive placebo tablet) – with about 1,000 women in each group who were asked to use their assigned study product every day. In early October 2011, VOICE stopped testing the oral tenofovir tablet because a routine review of study data by the trial’s independent data safety and monitoring board (DSMB) determined that although the oral tenofovir tablets were safe, they were no better than a placebo in preventing HIV in the women assigned to that study group.
More recently, on 17 November 2011, another routine DSMB review determined that tenofovir gel was safe but not effective in VOICE. Consequently, both gel arms (tenofovir gel and placebo gel) are being dropped from the study.
While finding that both the oral tenofovir tablets and tenofovir gel were not effective is very disappointing, the VOICE study has already answered two key questions it was designed to ask. After the study has been completed and all of the data have been analyzed, we will know whether daily use of the Truvada tablets was safe and effective in preventing HIV. The study’s final results will also help us to understand why the tenofovir tablets and tenofovir gel were not effective in the women enrolled in VOICE.
6. What is a DSMB and how does it relate to VOICE?
A Data and Safety Monitoring Board, or DSMB, is an independent group of clinical research experts, statisticians, ethicists and often community representatives that provides additional oversight to a clinical study. A DSMB regularly reviews blinded data that are not available to the investigators or anyone else, while a clinical trial is in progress. Based on its review of interim data, a DSMB may, at any time, recommend that a trial, or part of a trial, be stopped if there are concerns about safety, compelling evidence for a product’s effectiveness or if it is clear that a product is not effective in the study’s participants, a concept called futility. Before a trial begins, study teams define the specific “stopping rules” that would cause the study to close for efficacy, harm or futility.
Regular reviews of VOICE are conducted by the U.S. National Institute of Allergy and Infectious Diseases (NIAID) Prevention Trials DSMB, which makes its recommendations to the director of NIAID, Anthony Fauci, M.D., who decides whether to accept the DSMB’s recommendations. The DSMB for VOICE is composed of representatives from the U.S. and non-U.S. countries, including in Africa, who are independent of the study investigators, pharmaceutical sponsor and funding agency, and have no conflicts of interest in the outcomes of the studies reviewed. Since the study began in September 2009, the NIAID Prevention Trials DSMB has conducted six periodic reviews – in December 2009, June 2010, December 2010, May 2011, September 2011 and November 2011. All reviews prior to the September 2011 review indicated no concerns, and the DSMB recommended each time that the study continue, without changes.
At the16 September 2011 review, however, the DSMB recommended that VOICE stop evaluating the oral tenofovir tablet, because it would not be possible for the study to show a difference in effect between the tenofovir tablet and the placebo tablet (futility). The DSMB made clear in its recommendations that it had no concerns about the safety of any of the study products, including the oral tenofovir tablet. It also made clear at that time that VOICE should continue to evaluate the safety and effectiveness of tenofovir gel and the oral Truvada tablet. The DSMB then confirmed that the next routine review of VOICE study data would take place according to schedule in mid-November.
7. What was the outcome of the 17 November DSMB review?
The DSMB review on 17 November 2011 was the study’s fourth review of safety data and the third and final interim review of efficacy data – an assessment of the number of HIV infections that have occurred in each of the different study groups since the study began. Based on this interim review, which included study data for the period between 9 September 2009, when the study began, and 30 September 2011, the DSMB recommended that VOICE stop evaluating tenofovir gel, because there was no difference in effect between the tenofovir gel and placebo gel in preventing HIV infection among the women in those two groups. The HIV incidence rates in the two groups were nearly identical – 6.1 percent in the placebo gel group and 6 percent in the tenofovir gel group. HIV incidence represents the number of new infections that occur in a population over a specific period of time. In the case of VOICE, this means that for every 100 women in the gel arms, six acquired HIV in the course of a year. Because this evidence met the study’s criteria for futility under its stopping rules, the DSMB recommended that the women randomized to the tenofovir gel and placebo gel groups discontinue their use of the study product and be exited from the study as soon as it would be feasible to do so. The DSMB had no major concerns about the safety of the Truvada tablets, which it recommended the study team continue evaluating until the study’s natural conclusion.
8. Why did the DSMB meet so soon after its 16 September review of VOICE?
Well before VOICE even began, the research team had specified in the study’s protocol that three interim reviews of efficacy data would take place around specific time points as the trial was ongoing, i.e., when it would be expected that the study would reach 25 percent, 50 percent and 75 percent of its target HIV endpoints, respectively. An HIV endpoint is counted for each woman who acquires HIV during the trial, and the study ends when that target number is reached. VOICE was designed with the expectation that 217 HIV infections would occur within one year from the time enrollment was completed, which was June 2011. Based on prior statistical calculations, it had long been anticipated that the DSMB’s 75 percent-endpoint review would likely take place in mid-November 2011.
9. How is VOICE important?
VOICE is an important trial for understanding what works or doesn’t work for preventing HIV in women. Already, VOICE has answered two key questions it was designed to ask, finding that both the oral tenofovir tablets and tenofovir gel used daily were not effective in preventing HIV among the women in the study. The research team gratefully acknowledges the significant contributions that each and every VOICE participant – all 5,029 –have made in helping to answer these critical questions.
Following the promising results of CAPRISA 004, which found tenofovir gel 39 percent more effective than placebo gel when used before and after sex, VOICE took on added importance. The U.S. Food and Drug Administration indicated that along with the results of CAPRISA 004, it would review data from VOICE as the second pivotal trial to support possible licensure of tenofovir gel. However, instead of providing clear evidence of tenofovir gel’s efficacy, VOICE has provided clear evidence that the gel was not effective in the women in the study. Although disappointing, this information adds a critical dimension to discussions about the future of tenofovir gel.
Results of VOICE will be especially important for helping to understand the safety and effectiveness of oral Truvada in protecting against HIV in women. While two studies – Partners PrEP and TDF2 – showed that daily use of Truvada was very effective in both the men and women in those studies, it is not certain how generalizable the data from these two studies are to different groups of women. Partners PrEP involved men and women in committed relationships with an HIV-infected partner, in which both partners knew each other’s HIV status and both consented to enroll in the study. As such, the results may not represent single women, women with multiple partners or those who, though married, may not know whether or not her husband has HIV. In VOICE, participants may or may not have information about their partners’ HIV infection status, and may not even be in a steady relationship with a single partner. For example, many women enrolled in VOICE are unmarried. While the results of TDF2 suggest that Truvada was effective in both men and women, few conclusions can be drawn from the results concerning the effectiveness of Truvada specifically in women due to the small numbers of women who became infected during follow-up. The one trial besides VOICE that involved only women, a study called FEM-PrEP, was not able to demonstrate that daily use of Truvada was effective in that study population, women considered higher-risk. Higher risk women included those who engage in frequent sexual intercourse or have more than one sex partner. A full analysis of all the study information, which is expected to be available at the end of 2011 or early 2012, is needed before knowing what factors might have contributed to FEM-PrEP’s outcome. In the meantime, VOICE remains perhaps the one trial that can help determine whether Truvada holds promise for protecting women against HIV.
Women account for 60 percent of adults with HIV in sub-Saharan Africa, where unprotected heterosexual intercourse is the primary driver of the epidemic. Young women are especially vulnerable. In southern Africa, young women are up to five times more likely to become infected with HIV than young men and more than a quarter (26 percent) of all new global HIV infections are among women aged 15-24. Women are twice as likely as their male partners to acquire HIV during sex. Although correct and consistent use of male condoms has been shown to prevent HIV, women are not always able to negotiate their use, so women need methods for preventing HIV that they can control themselves.
10. What happens next? When and how will this latest change be implemented at trial sites?
All VOICE participants are being informed about this latest modification to drop the two gel arms. Because the DSMB had no concerns about the safety of tenofovir gel, participants randomized to the vaginal gel arms will discontinue use of their assigned study product at their next scheduled clinic visit, which is likely to be in December or January. According to the normal process for women ready to exit the study, the women in the two gel groups will then return to the study clinic for a last set of tests and procedures, including HIV testing and counseling, eight weeks after stopping their assigned study product. During this last study visit, the participants will be “unblinded” and learn which group they had been randomized to. They will also be provided information about where they can receive HIV testing and counseling, contraception and other medical or support services as needed. Women who became HIV-infected and/or pregnant during VOICE, and subsequently enrolled in an MTN ancillary study (MTN-015 for those who acquired HIV, and MTN-016 for those who became pregnant), may continue to participate in these studies.
11. What progress has been made in implementing the close of the tenofovir tablet arm?
The process of discontinuing the use of the tenofovir tablets among the participants in that study group has been proceeding in an orderly fashion. Beginning 3 October 2011, women identified as having been assigned to use the oral tenofovir tablets were told to discontinue use of the tablets when they came for their monthly scheduled clinic visit. As of 15 November, most of the women in that group have stopped taking the tenofovir tablet. Researchers expect that all the women in the oral tenofovir arm will have completed their eight-week follow-up visit and exited the study by the end of January.
12. Are you disappointed about this outcome, having now to drop yet another arm of VOICE?
The DSMB acted in accordance with its charge to ensure that the clinical trials it reviews are conducted ethically and with the highest regard for the safety and wellbeing of study participants. While the study team is disappointed in the outcome, it respects the process by which this decision was made and recognizes that the HIV incidence that was observed in women in the tenofovir gel and placebo gel clearly meets the determination of futility. VOICE was designed to answer key questions about the safety and effectiveness of oral tenofovir tablets, oral Truvada tablets and tenofovir gel for preventing the sexual transmission of HIV among high-risk women in Africa. So it is important to recognize that the study – and the women who have been taking part in VOICE – have already succeeded in answering the study’s questions about oral tenofovir tablets and tenofovir gel: neither was effective in VOICE. The goal now is to complete VOICE and determine whether Truvada tablets are effective in preventing HIV in these women, and to understanding why the tenofovir tablets and tenofovir gel were not effective.
13. How could tenofovir gel not be effective in VOICE when the CAPRISA 004 study found it was?
The CAPRISA 004 study found tenofovir gel was 39 percent more effective than placebo when used before and after sex, a finding that was considered a major milestone for the field. At the same time, it is important to consider the study’s demographics and the results for what they are. The CAPRISA 004 study involved 889 women, who were all from KwaZulu-Natal, South Africa, and coming from just one study and involving a single population, the result was not considered strong enough, especially for supporting possible product licensure. And while the 39 percent level of effectiveness was the result most often referenced, it’s important to understand that the study also found that the true level of effectiveness of tenofovir gel – when used before and after sex – could be anywhere between 6 and 60 percent.
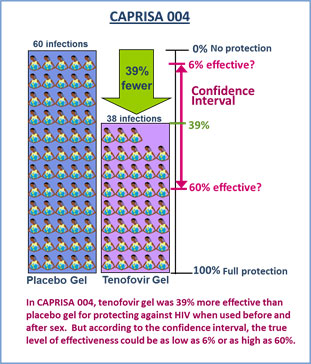
Of the 889 women in CAPRISA 004, 98 women acquired HIV during the study – 60 women in the placebo gel group and 38 in the tenofovir gel group. This means that there were 39 percent fewer infections among those assigned to use tenofovir gel compared to those in the placebo gel. Because 39 percent is a figure that represents only an estimate of what the true effectiveness may be, it should be considered in the context of another statistical measure called a confidence interval that indicates where the true result lies. The confidence interval for CAPRISA 004 had a lower boundary of 6 percent and an upper boundary of 60 percent.
To get closer to the truth about any product’s effectiveness, more than one study is almost always required. As such, to be certain that tenofovir gel is effective, additional data from more women was needed.
There were more than 2,000 women in the two gel arms of VOICE. Some of these women were also from KwaZulu-Natal, but there were women from other parts of South Africa, and Zimbabwe and Uganda as well. Although not all the data are available at this time, we know that in VOICE the HIV incidence rates in the two groups were nearly identical – 6.1 percent in the placebo gel group and 6 percent in the tenofovir gel group. This suggests that the level of effectiveness for tenofovir gel in VOICE is likely to be near the lower end of the range estimated from CAPRISA 004. As such, the outcome of VOICE is not inconsistent with the results of the CAPRISA 004 study.
Like CAPRISA 004, VOICE was a well-designed and well-conducted study. It’s for this reason that VOICE has already been able to answer one of the questions it was designed to ask –tenofovir gel was not effective in the women in the study, who were asked to use the gel every day. While this may be viewed as a disappointment, the study has succeeded in providing an answer nonetheless, and the VOICE team is very grateful to the women who have participated in the trial, who have made this achievement possible. After the study is completed, a full analysis of all data will take place. Only then will it be possible to understand why tenofovir gel was not effective in VOICE.
14. What are the differences between VOICE and CAPRISA 004, and also the FACTS 001 study?
CAPRISA 004 was a Phase IIb trial conducted by the Centre for the AIDS Programme of Research in South Africa (CAPRISA) that assessed the safety and effectiveness of tenofovir gel among women who applied it within 12 hours prior to sex and as soon as possible within 12 hours after sex. The study was conducted at two sites in the KwaZulu-Natal province of South Africa and involved 889 women. As a follow-up to CAPRISA 004, FACTS 001 is a larger study of the same regimen that is being spearheaded by a South African-based research consortium. FACTS 001 is designed as a Phase III study that plans to enroll a minimum of 2,200 women at nine sites in South Africa. Unlike the CAPRISA 004 and FACTS 001 studies, VOICE was designed to evaluate daily use of tenofovir gel, regardless of when participants have sex, and involves women not only from South Africa, including from KwaZulu-Natal, but also Uganda and Zimbabwe.
15. Why did VOICE choose to test tenofovir gel used daily?
VOICE was designed according to current understanding about the mechanisms of tenofovir. As such, researchers conducting VOICE believed that using gel every day would provide cells with a sufficient level of drug needed for continuous protection. For the drug to work against HIV, it must be in its activated form, which requires that it first get inside a target cell and then add two protective molecules, called phosphates, to its structure. It takes tenofovir a bit of time to forge this shield, and the protection it offers gradually subsides. Researchers had seen daily dosing as a way to supply cells with a sustained defense against HIV.
16. Was tenofovir gel not effective in VOICE because of poor adherence, because women simply weren’t using the gel?
We won’t understand the reasons why tenofovir gel was not effective in the women in VOICE until after the study has been completed and we have done a full analysis of data from all study groups. We are continuing to evaluate the safety and effectiveness of the oral Truvada tablet in preventing HIV and expect to complete all follow-up of participants by end of July. Study results are expected to be available late 2012 or early 2013.
17. How is adherence measured in VOICE?
Throughout the study, VOICE researchers ask participants a series of standard questions about sexual activity, product use, product use adherence, male condom use and product sharing. Participants also answer the same kinds of questions privately with the help of a computer, an approach that is thought to be a better way to collect sensitive information. In addition, blood and vaginal fluid samples are taken from participants at different times in the study will help determine how well participants followed the study regimens by measuring the amount of drug present. VOICE trial sites with laboratory capacity are also collecting blood for the purposes of analyzing drug levels in a type of blood cell called peripheral blood mononuclear cells (PBMCs), which may be an especially reliable measure of product use. Provided that sites receive Institutional Review Board and/or Ethics Committee approval, small samples of hair will also be collected for analysis of drug levels from participants who provide separate consent.
18. What does VOICE do to protect the safety of participants?
VOICE includes numerous measures to monitor and protect the safety and wellbeing of participants, including interim reviews of data by an independent group of clinical research experts, bioethicists and statisticians called a data safety monitory board, or DSMB. The study team also works actively to decrease participants’ risk of HIV infection by providing free condoms, regular counseling about preventing HIV and other sexually transmitted infections (STI), and STI testing and treatment. Safety data are reviewed regularly by a team of clinicians with expertise in infectious diseases, obstetrics and gynecology and internal medicine. As with any study, significant concerns about participant safety in VOICE would prompt the study team to take immediate steps to stop participants from using the study products.
19. Will VOICE tell us anything about tenofovir gel and HSV-2?
An unexpected finding of the CAPRISA 004 study was that tenofovir gel helped to protect against new cases of genital herpes (HSV-2). VOICE researchers are hopeful that data collected in the women enrolled in their study will provide additional information about the potential effectiveness of tenofovir gel for preventing HSV-2 infection.
20. What is the VOICE B Bone Density Substudy?
VOICE B is a substudy of VOICE that is focused on determining the potential effects, if any, oral ARV tablets have on bone health in HIV-negative women. VOICE B is being conducted at VOICE sites in Uganda and Zimbabwe in a subset of VOICE participants who were randomly assigned to one of the oral tablet regimens. Because VOICE is continuing with the Truvada tablet and placebo tablet arms of the study, those women who have also enrolled in VOICE B will remain in that study, and undergo bone density scans at six-months and a year after they exit the parent study. Importantly, women in the tenofovir tablet group who were also participating in VOICE B will be allowed to re-enter the substudy. VOICE B will provide valuable data that will help inform communities looking at the promise and potential rollout of ARV-based approaches for prevention of HIV in both men and women.
21. What do we know about Truvada for preventing HIV?
The results of a study called iPrEx, reported in November 2010, provided the first evidence that oral PrEP can help prevent HIV. iPrEx found Truvada – together with a comprehensive HIV prevention package – was safe and 44 (43.8) percent more effective than a placebo tablet for protecting against HIV in men who have sex with men. iPrEx-OLE, an open-label extension trial of Truvada in men who have sex with men (MSM), is getting underway at trial sites in South America, the U.S., Thailand and South Africa. It follows the iPrEx Study, which found daily use of oral Truvada reduced the risk of HIV by 44 percent compared to a placebo among MSM.
In the Partners PrEP Study, researchers from the University of Washington and their collaborators in Uganda and Kenya, evaluated the safety and effectiveness of daily use of two ARVs – tenofovir and Truvada – among men and women in a committed relationship with a partner who is HIV-positive. The study enrolled 4,758 serodiscordant couples. The results, reported in July 2011, provide the strongest evidence yet in favor of oral PrEP, with 62 percent fewer HIV infections among participants assigned to take the ARV tenofovir daily compared to participants who took a placebo tablet, and 73 percent fewer infections among those who took Truvada. Similarly, the TDF2 Study, a smaller study that involved 1,200 heterosexual men and women in Botswana, found that 62.6 percent fewer HIV infections had occurred in the group assigned to take Truvada than in the placebo group.
In April 2011, researchers from the FEM-PrEP study announced the trial would be closing earlier than planned because an interim review by its data monitoring committee determined that the study could not show that Truvada was effective in its population of women. The FEM-PrEP Study was a placebo-controlled Phase III trial that had enrolled 2,119 higher-risk women between the ages of 18 to 35 in Kenya, Tanzania and South Africa. A full analysis of all the study information, which is expected to be available at the end of 2011 or early 2012, is needed before knowing what factors might have contributed to FEM-PrEP’s outcome.
Possible Implications
22. There has been a lot of talk about possible licensure of tenofovir gel. How will the outcome in VOICE affect the future of tenofovir gel as a product for preventing HIV in women?
Following the announcement of the results of CAPRISA 004 in July 2010, expectations had been especially high that success would be replicated in a second trial, such as VOICE, and discussions concerning its possible approval and introduction had already begun in earnest. Regulatory agencies in the United States and South Africa had agreed to review data from CAPRISA 004, the VOICE trial, and the FACTS 001 trial, along with data from related studies, in order to determine whether tenofovir gel should be licensed for use. Specifically, the U.S. Food and Drug Administration had previously indicated that it would base its decision whether to approve the gel primarily on two pivotal trials: CAPRISA 004 and VOICE.
As a co-licensee for tenofovir gel, CONRAD, of Arlington, Virginia, has been leading all discussions with drug regulatory authorities and working to ensure that individual requirements are met. In June 2011, CONRAD and the South African government’s Technology Innovation Agency (TIA) announced a license agreement that grants TIA the rights to manufacture and distribute tenofovir gel in Africa if it is proved effective in current trials and subsequently receives regulatory approval.
In VOICE study participants, tenofovir gel was safe but it clearly was not any better than a placebo in preventing HIV infection, a finding that adds a critical dimension to the discussions about the future of the gel.
We cannot know what the FDA or the South African Medicines Control Council will decide, or what the product’s developer, CONRAD, and its partners plan to do with this new information from VOICE. However, the FACTS 001 study’s sponsors have indicated that the trial will continue unchanged. FACTS 001 began enrolling women at trial sites in South Africa in October. The study, which plans to enroll at least 2,200 women, is testing the same regimen as in CAPRISA 004, in which women used the gel before and after sex.
23. With the tenofovir gel arm in VOICE now closing, does this mean that the FACTS study will be stopped?
FACTS 001 is a Phase III trial that seeks to replicate the results of CAPRISA 004, which found tenofovir gel applied before and after sex reduced the risk of HIV by 39 percent compared to placebo. FACTS 001 enrolled its first participant in October 2011 and plans to enroll a minimum of 2,200 women at nine sites in South Africa, with results available in 2014. According to the study’s funders – the South African Department of Science and Technology, the United States Agency for International Development (USAID), the South African Department of Health and the Bill & Melinda Gates Foundation – there are no plans to stop or pause the study in response to the outcome in VOICE.
24. How do these changes affect CHOICE?
CHOICE – Committed to Having Options for Interventions to Control the Epidemic, or MTN-018, is a follow-up open-label study to VOICE, which was designed to move forward if VOICE were to find any of the products safe and effective. Based on two interim reviews of study data, VOICE has already determined that tenofovir gel and tenofovir tablets were not effective in VOICE. If the results of VOICE indicate that Truvada is safe and effective, all former participants who are HIV-negative will be invited to join and have access to the study product during the one-year study. Women who are pregnant, or intend to become pregnant, or are breastfeeding, will be able to participate in sub-studies of CHOICE that plan to investigate product safety among pregnant and breastfeeding women. If implemented, CHOICE will help to inform the broader implementation of oral PrEP and be important for understanding the overall safety of this approach in healthy women of reproductive age.
25. MTN is conducting other tenofovir gel trials and has plans for others. What happens now?
MTN leadership, along with its NIH funders, is considering how the VOICE DSMB outcome will impact other tenofovir gel-related trials that are in progress, or those that were anticipated to be starting soon. Some of these studies, such as those involving pregnant and breastfeeding women or adolescent girls, could still be of great value as long as the FACTS 001 study continues and there remains an interest in developing tenofovir gel as an HIV prevention product. Data from some of these studies could also help support a possible indication to prevent genital herpes (HSV-2). Because the CAPRISA 004 study had the unexpected finding that tenofovir helped to protect against new cases of HSV-2, the FACTS 001 study is looking to evaluate the gel’s safety and effectiveness for preventing HSV as well. Additional data from MTN studies could be useful in the regulatory review process.
26. How does closing the tenofovir gel arm in VOICE affect the use of tenofovir gel as a potential rectal microbicide?
The MTN has just completed a Phase I study that evaluated the safety of tenofovir gel reformulated for rectal use. Depending on the results, which are expected early next year, researchers were planning to conduct a Phase II study testing the safety and acceptability of the reformulated gel used daily or before sex by men who have sex with men (MSM) at trial sites in Peru, South Africa, Thailand and the United States. So, unlike VOICE, the rectal studies are focused on a different population of high-risk individuals in whom HIV is acquired through anal sex rather than vaginal sex. Tenofovir gel may work differently against HIV in rectal tissue. MTN researchers will decide whether to proceed with the Phase II trial after reviewing the study data from the Phase I trial.
27. Where does ARV-based HIV prevention go from here?
ARV-based prevention methods – as either a vaginal microbicide or an oral tablet – remain promising approaches. And there is good evidence from other studies, most notably, Partners PrEP and iPrEx, that ARVs do indeed work in preventing HIV. Moreover, ARVs are already used effectively in preventing mother to child transmission. ARV-based microbicides may still prove to be promising, but it will be some time before we have a more complete picture about ARV-based approaches in women. The FACTS 001 study of tenofovir gel has just started, and another trial of a vaginal ring containing the ARV dapivirine will begin the middle of next year.
Other ARVs are being explored as vaginal microbicides. These include dapivirine and maraviroc. But until clinical trials are completed we will not know if they will be safe and effective. ASPIRE – A Study to Prevent Infection with a Ring for Extended Use, also known as MTN-020, is a large Phase III study of a vaginal ring containing dapivirine that will start the middle of next year. ASPIRE is the first Phase III trial of a vaginal ring for HIV prevention and the first study to evaluate the effectiveness of a long-acting product intended for extended use. The International Partnership for Microbicides (IPM), a non-profit product development partnership based in Silver Spring, Maryland, developed the ring. As part of its strategy to license the dapivirine ring, IPM will conduct The Ring Study (IPM 027) in parallel with the ASPIRE, and collect long-term safety and efficacy data. Results of both studies are expected late 2014 or early 2015.
28. What is ASPIRE?
ASPIRE – A Study to Prevent Infection with a Ring for Extended Use, also known as MTN-020, is a Phase III study that seeks to determine whether a woman’s use of a vaginal ring containing the antiretroviral (ARV) drug dapivirine is a safe and effective method for protecting against HIV infection. Vaginal rings are products designed to allow for the slow delivery of a drug or multiple drugs to cells inside the vagina over a period of weeks or months. As a potential method for preventing sexual transmission of HIV, rings are seen as an alternative to microbicide gels tested in clinical trials that are used every day or at the time of sex. The dapivirine ring being tested in ASPIRE is designed to be replaced every four weeks. MTN researchers plan to begin the trial in June or July 2012.
The study, which is designed to provide the strength of evidence to support potential licensure of the dapivirine vaginal ring, will enroll approximately 3,476 women at several sites in at sites in Malawi, Uganda, South Africa, Zambia and Zimbabwe. The International Partnership for Microbicides (IPM), a non-profit product development partnership based in Silver Spring, Maryland, developed the ring. As part of its strategy to license the dapivirine ring, IPM will conduct The Ring Study (IPM 027) in parallel with the ASPIRE, and collect long-term safety and efficacy data. Results of both studies are expected late 2014 or early 2015.
# # #
More information about the VOICE Study and related topics are available at, http://www.mtnstopshiv.org/news/studies/mtn003. A summary of recent trial results of other PrEP studies can be found at http://www.avac.org/ht/d/sp/i/326/pid/326.
Click here for PDF version of this document.
25-November-2011
See
